Ice melt blends are rapidly becoming a growing segment of the ice melt market. But…
Have you ever wondered how an ice melt works or what makes salt melt ice? Well, let’s start with some basic chemistry on the composition of water and what causes it to freeze and thaw in the first place.
Water
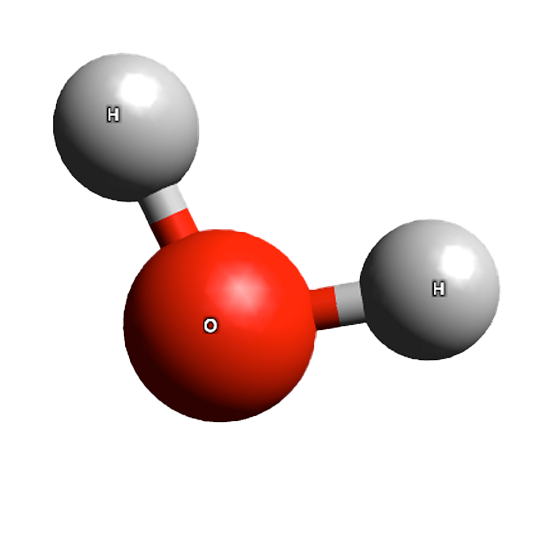
H2O, water, or dihydrogen monoxide, is a molecule composed of one oxygen atom and two hydrogen atoms. These atom are held together by a covalent bond. Due to an unequal sharing of the electrons between the atoms, the water molecule has a slight polarity. The oxygen end of the molecule has a negative charge while the hydrogen end has a positive charge. (Think of the water as a little stick magnet, the two ends having different charges.)
Due to these charges, water is always seeking to align itself with other water molecules around it. Now remember, opposites attract. This causes water to arrange itself in repeating patterns with the hydrogen from one molecule lining up with the oxygen of another molecule and so on.
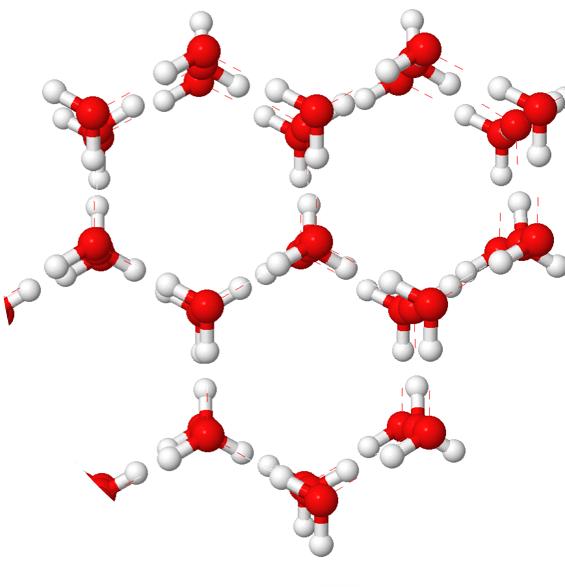
However, these charges are relatively weak and, at temperatures above 32°F, are not strong enough to contain the energy the molecules have. This results in close-held liquid that does not have any set pattern or structure, also known as water. At temps above 212°F, these charges have no control over the molecules and they become a gaseous vapor know as steam. But, when the temp drops below 32°F, the molecules become controlled by the opposing charges and arrange themselves in a repeating hexagonal pattern, known as ice.
Salt
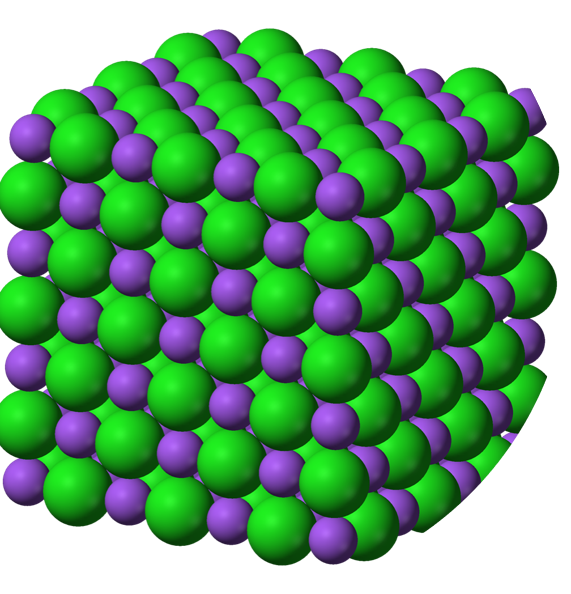
Salt (whether sodium chloride, magnesium chloride, or calcium chloride) is usually composed of two different ions held together by ionic bonds. These ionic bonds work by strong opposite charges between the different ions.
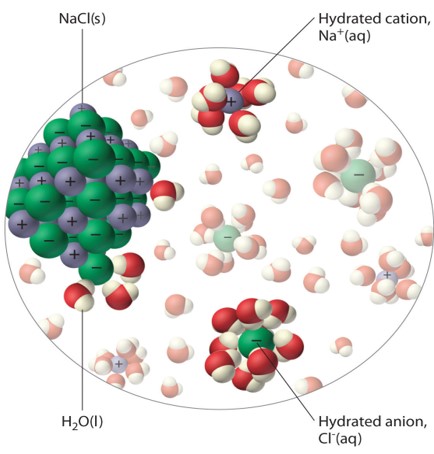
When a salt is placed into water, (remember that water contains charges just like the salt ions), the charges of the water molecules align themselves with the strong opposing charges of the salt ions. This causes the water to surround the individual ions of the crystal. Once enough molecules have surrounded the ions, the pull from the charges of the water becomes greater than the charges holding the ions together, breaking the ions apart (dissolving). The water will continue to dissolve the crystal in this manner until either there is nothing left to dissolve or until all the water molecules are already surrounding an ion in the water and there is no free molecules left to continue the dissolving process.
How Does That Melt Ice?
I’m glad you asked!! So, before any salt was added, the only thing affecting the ability of the water to freeze was the energy of the water. When the energy level became low enough, water was able to arrange itself into ice. Now, not only does a high energy keep the water from bonding to itself, but you also have salt ions that are working to disrupt the attraction of the water for itself. Therefore, the salt water requires an even lower energy level (temperature) in order to freeze. As a rule of thumb, the more ions added to the water, the greater the effect on freezing point.
So there you have it, that is the science behind how an ice melt works in a nutshell. Obviously, there is a whole lot more that goes into play that I did not mention. My goal was to simply give you a brief explanation as to how salt can affect the freezing point of water. Hopefully this has been educational for you and was able to answer any questions you may have had!

